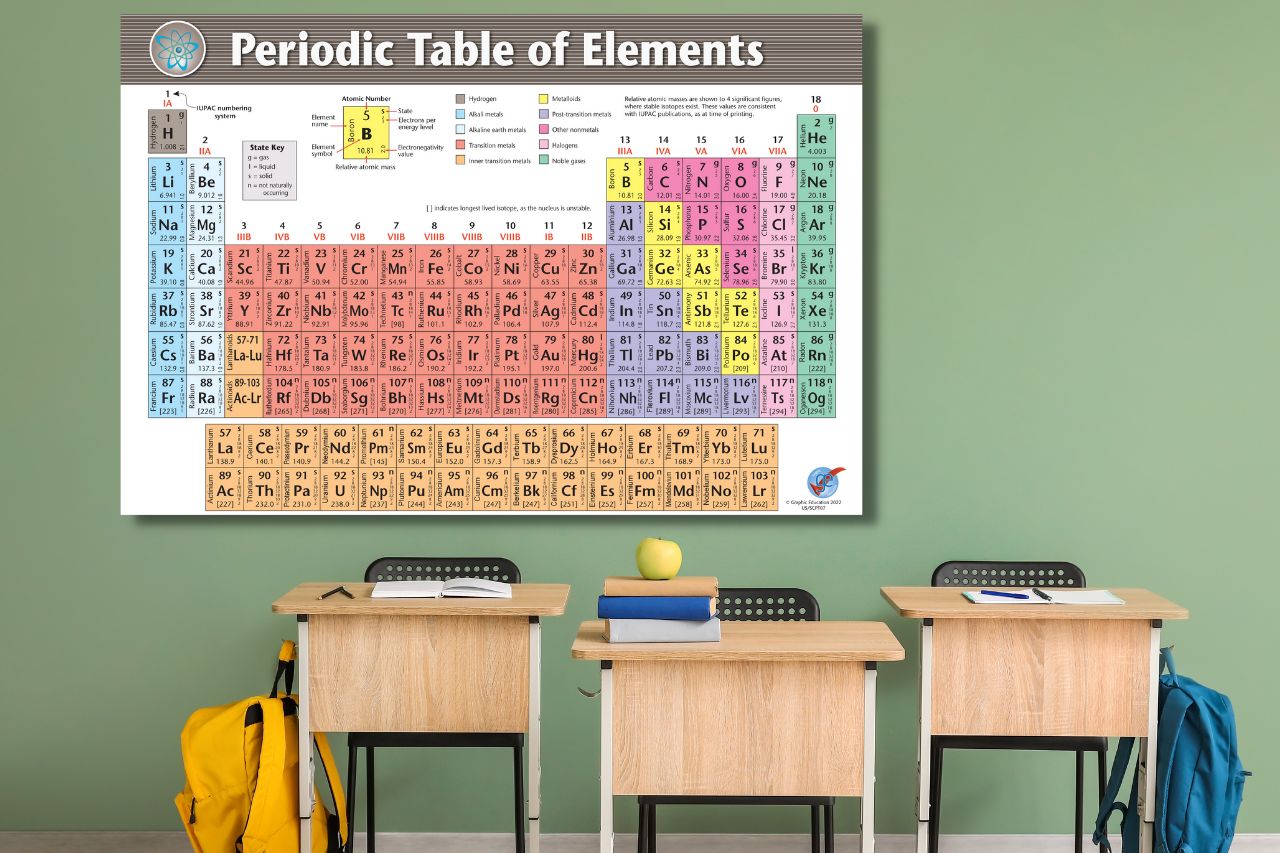
The periodic table – a remarkable scientific treasure trove, cataloging the building blocks of our universe. With each element meticulously arranged, it represents the culmination of centuries of scientific research, discovery, and collaboration. At the heart of our quest for knowledge, the International Union of Pure and Applied Chemistry (IUPAC) has meticulously crafted a periodic table that is not only accurate but also up-to-date. This article delves into the legitimacy and quality of the information used in our high-quality periodic tables, which are based on data sourced directly from IUPAC. So, let's embark on a journey through the intricate process of maintaining this indispensable scientific tool.
The IUPAC Standard: A Testament to Precision and Collaboration
Our periodic tables are based on the latest release of IUPAC's Periodic Table of the Elements and Isotopes (IPTEI). This release, dated 4 May 2022, includes the most recent abridged standard atomic weight values released by the IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW), compiled as part of the 2021 Table of Standard Atomic Weights 2021. With IUPAC's involvement in various aspects of the periodic table, we can assure you that the information presented in our product is legitimate, accurate, and up-to-date.
The Pillars of IUPAC's Involvement in the Periodic Table
IUPAC's role in maintaining the periodic table is multifaceted, involving numerous aspects such as:
- Establishing criteria for a new element discovery
- Defining the structure of temporary names and symbols
- Assessing claims resulting in the validation and assignation of an element discovery
- Coordinating the naming of a new element, involving the research laboratory and allowing for public comments
- Setting up precise rules for naming new elements
- Defining Group 1-18 and collective names
- Determining which elements belong to Group 3
- Regularly reviewing standard atomic weights
These crucial responsibilities ensure that the information contained within our periodic tables is both accurate and reliable, enabling scientists and educators worldwide to rely on its content.
The Rigorous Process of Element Discovery, Validation, and Naming
IUPAC's stringent guidelines for the discovery, validation, and naming of new elements ensure that the periodic table remains an authoritative source of information. This involves a rigorous process, including assessing if an element has been "discovered," establishing criteria for new element discoveries, setting up temporary names and symbols, and validating and assigning element discoveries. Moreover, IUPAC also coordinates the naming process, inviting laboratories to propose names and symbols for newly discovered elements, reviewing the proposals, and eventually formalising the names after public review.
The IUPAC Periodic Table: A Living Document
With its direct involvement in various aspects of the periodic table, IUPAC ensures that it remains a living document – one that evolves with the ever-growing body of scientific knowledge. The organisation regularly reviews standard atomic weights and updates the periodic table, providing you with the latest and most accurate information. As a result, our periodic tables remain up-to-date, reflecting the current state of scientific understanding.
In summary, the legitimacy and quality of the information used in our high-quality periodic tables are the direct result of IUPAC's unwavering commitment to precision, accuracy, and collaboration. Our periodic tables are not just products; they are the embodiment of centuries of scientific inquiry and an ongoing quest for knowledge. By using our periodic tables, you are not only embracing the rich legacy of scientific discovery but also becoming a part of the vibrant global community dedicated to science.